PD1 and PDL1 inhibitors, collectively referred to as PDx, have revolutionized cancer treatment since the approval of pembrolizumab, commonly known as Keytruda®, for melanoma in 2014. Since then, 7 more PDx-targeting drugs have been approved. However, not all patients benefit from them, and some develop resistance to the treatment.
As part of our ongoing initiative to champion innovative discoveries in immunotherapy and keep the scientific and medical community informed about the latest developments in the field, we regularly publish review articles that provide comprehensive, independent, in-depth analyses of various topics related to cancer immunotherapy. Our latest addition to this series is an overview of the most recent developments in PDx trials. For the first time, this review includes a comprehensive analysis of the inclusion and exclusion criteria in PDx trials to investigate the types of studies that enroll patients previously treated with PDx and have developed resistance to this modality.
What’s new in PDx therapy development?
PDx trials, which involve the use of PD1 and PDL1 inhibitors in cancer treatment, have reached a turning point in 2022. For the first time we observed a small decline in the number of new trials. PDx treatment has evolved towards using alternative approaches to monoclonal antibodies, such as bispecific antibodies, small molecules, and oncolytic viruses. Additionally, combinations with other immunotherapies and targeted therapies are becoming more prominent in ongoing clinical trials. The choice of a specific combination, however, depends on the type of cancer being treated.
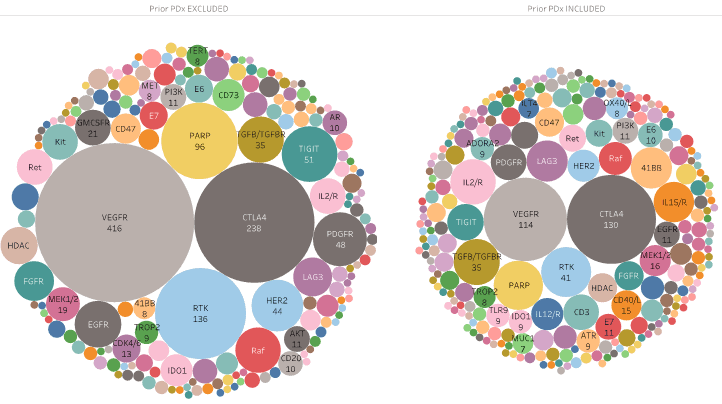
In recent cancer trials, there is an increasing trend in including patients who have previously received PDx (PD1 and PDL1 inhibitors) treatment, in an effort to focus on finding efficient therapeutic combinations to increase responsiveness to PDx therapy. Various cancer indications and combinations of treatments are being explored for PDx-resistant patients, with a focus on other immune-oncology (IO) combinations, particularly those that target immune cells other than T-cells or utilize oncolytic viruses. Furthermore, more trials are testing PDx therapies as first-line treatments rather than as a second-line option after other treatments have been attempted.
In conclusion, PDx remains a cornerstone of cancer immunotherapy and a ray of hope for patients. However, primary and acquired resistance to PDx is a pressing concern. Ongoing clinical trials are paving the way for the discovery of new therapy combinations for patients. We will continue to monitor these advances and keep our readers updated on the latest developments in the field of PDx therapy and immuno-oncology as a whole.
Please follow our landscape series, which covers diverse topics related to immune-oncology, and support our efforts to bring innovative immune therapies to patients more quickly. Together, we can create a world immune to cancer.
To read the full summary go to PD1/PDL1 clinical trials adapt to a growing landscape of patients resistant to PDx (nature.com)